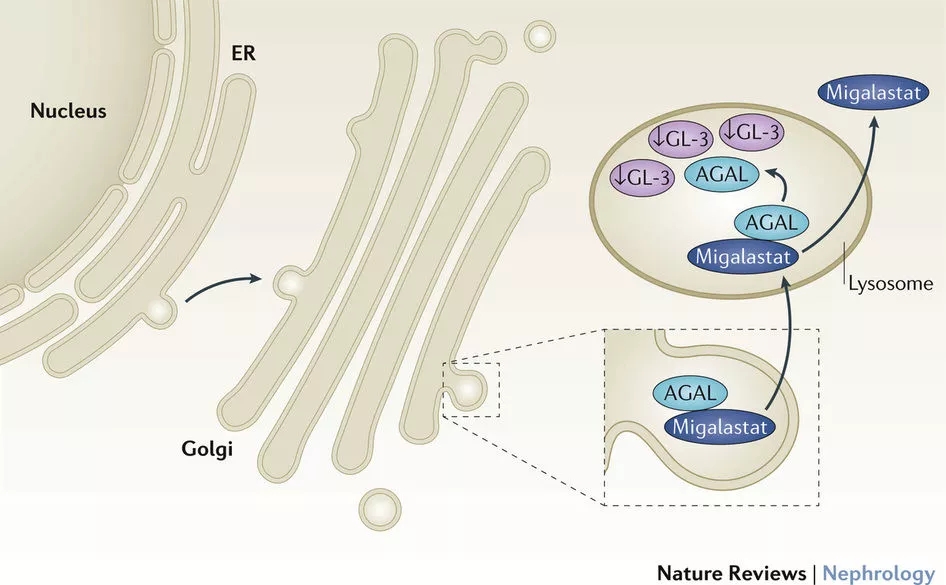
Amicus submits the first US new drug listing application December 15, 2017 Source: WuXi PharmaTech Today, WuXi PharmaTech partner Amicus Therapeutics submitted a new migalastat marketing application to the US FDA for the treatment of Fabry disease patients with specific mutations 16 years of age and older. If all goes well, this new drug is expected to be approved for listing in 2018. Fabry disease is a hereditary disease that is most common in the United States. The cause of this disease is the lack of α-galactosidase (often caused by mutations in the GLA gene), which can cause problems in the metabolism of patients, leading to special lipids such as GL-3 in the heart, kidney, central nervous system, and skin. It accumulates in tissues, causing a series of problems such as pain, kidney failure, heart disease, and stroke. ▲Migalastat's mechanism of action (Source: "Nature Reviews Nephrology") The migalastat brought by Amicus can solve the problem of patients. The new drug is an innovative treatment that stabilizes alpha-galactosidase, a dysfunctional dysfunction in patients with specific mutations, allowing these enzymes to restore their ability to clear harmful substrates. It is estimated that this new drug can help 35%-50% of patients with Fabry disease worldwide. In two key phase 3 clinical trials, the researchers tested the efficacy of migalastat in patients who were initially treated or switched to enzyme replacement therapy and obtained excellent data. To this end, the new drug has been marketed in Europe for first-line treatment of patients with Fabry disease who are over 16 years of age with appropriate mutations. The listing application submitted to the US FDA is based on all existing clinical trial data. Previously, it was granted orphan drug status and fast track qualifications by the US FDA. ▲ Dr. John Crowley, President and CEO of Amicus (Source: Amicus Official Website) “Today marks the first new drug application we have submitted to the US FDA. This important milestone is based on the strong cooperation and commitment of patients, doctors, and Amicus employees. They have spent on the advancement of migalastat. For more than 10 years, Dr. John Crowley, President and CEO of Amicus, said: "After the launch of migalastat in Europe, our goal is to further improve the Fabry disease with the appropriate mutations in the US, Japan, and other regions. The patient used migalastat. We look forward to working with the FDA in the review process to get migalastat approved in 2018." We hope that this new drug will soon come to the world of patients with Fabry disease and improve their lives. Reference materials: [1] Amicus Therapeutics Submits New Drug Application to US FDA for Migalastat for Treatment of Fabry Disease [2] Amicus official website Original title: Congratulations! Amicus submits the first US new drug listing application Hair Vitamin Serum Capsules, Hair Vitamin Capsules, Hair Growth Capsules Xi'an Tian Guangyuan Biotech Co., Ltd. , https://www.tgybiotech.com